Subscribe to get the latest news and updates.
Innovation Excellence is unleashed when ideas are spurred by action, making the impossible happen.
As a research-based pharmaceutical company, Zydus' Innovation programme is spearheaded by 1300 researchers across 19 sites, working on differentiated medicines for the future. From NCEs to vaccines, biosimilars and niche technologies, the group is exploring different ideas, concepts and continuously innovating.
The Zydus Research Centre (ZRC) is the dedicated research arm of the Zydus Group. With its team of over 400 research professionals, ZRC spearheads the group’s quest of creating healthier and happier communities globally. Spread over an area of over 4,75,000 sq ft, ZRC is working on cutting edge technologies in different scientific disciplines to discover novel therapeutic agents. The centre has capabilities to conduct drug discovery & development from concept to IND enabling preclinical and clinical studies.
In 2013, the group was the first to identify, develop and launch LipaglynTM (Saroglitazar) the novel drug to treat diabetic dyslipidemia – a global, unmet healthcare need. Lipaglyn is the first NCE from an Indian research pipeline to move from the lab to the market. It offers dual benefits of lipid and glycemic control in one single molecule. It is an innovation that has helped over 700000 people suffering from diabetic dyslipidemia in India lead healthier lives.
* Saroglitazar/ Bilypsa / Lipaglyn is a prescription drug authorised for sale in India only and can be taken only under the advice and guidance of a registered medical practitioner.
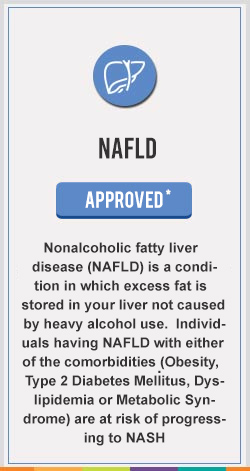
Saroglitazar Mg currently approved in India is a prescription medicine for the treatment of Hypertriglyceridemia, Diabetic Dyslipidemia in Patients with Type 2 Diabetes not controlled by statins, Type 2 Diabetes and Non-Alcoholic SteatoHepatitis (NASH) & Non Alcoholic Fatty Liver Disease (NAFLD) with comorbidities
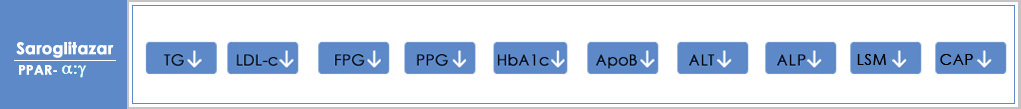
Saroglitazar Mg is an investigational new drug in the United States of America, and is currently being evaluated in Phase II clinical trials for the treatment of Non-Alcoholic SteatoHepatitis (NASH). Zydus has completed enrolment of Phase 2b/3 EPICS III trial of Saroglitazar Mg in patients with Primary Biliary Cholangitis (PBC). The USFDA has granted ‘Orphan Drug Designation’ and ‘Fast Track Designation’ to Saroglitazar Mg for Primary Biliary Cholangitis (PBC). Saroglitazar Mg has received Orphan Status from EMA for PBC.





* Saroglitazar is a prescription drug authorised for sale in India only and can be taken only under the advice and guidance of a registered medical practitioner.
* Saroglitazar/ Bilypsa / Lipaglyn is a prescription drug authorised for sale in India only and can be taken only under the advice and guidance of a registered medical practitioner.
Potential Indication |
 |
||||||||
Current Status |
|
||||||||
Unmet Need |
NLRP3 inflammasomes are involved in the inflammation process by production and release of proinflammatory cytokines IL-1β and IL-18. This harmful inflammation within the body leads to the onset and development of various kinds of diseases, including Acute Respiratory Distress Syndrome (ARDS), auto-immune diseases, inflammatory diseases, cardiovascular diseases, metabolic disorders, Gastro-intestinal diseases (inflammatory bowel disease), renal diseases and CNS diseases. Currently most of the inflammatory disease are mainly treated by several approved biological drugs. A novel oral small molecule NLRP3 inhibitor like ZYIL1 with good safety profile has the potential to be the new standard of care for several inflammatory disease. | ||||||||
Publications |
1. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Oral NLRP3 Inflammasome Inhibitor ZYIL1: First-in-Human Phase 1 Studies (Single Ascending Dose and Multiple Ascending Dose). Clin Pharmacol Drug Dev. 2022 Sep 5. doi: 10.1002/cpdd.1162. Epub ahead of print. PMID: 36065092. 2. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of ZYIL1 in Three Patients with Cryopyrin-Associated Periodic Syndromes, Clinical Pharmacology in Drug Development, 2023, 0(0) 1–8. DOI: 10.1002/cpdd.1318. 3. A novel selective NLRP3 inhibitor shows disease-modifying potential in animal models of Parkinson's disease. Brain Res. 2024 Jul 27;1842:149129. doi: 10.1016/j.brainres.2024.149129. |

The first drug to get approval for NAFLD & NASH anywhere in the world , an NCE from an Indian research pipeline to move from the lab to the market.
* Saroglitazar/ Bilypsa / Lipaglyn is a prescription drug authorised for sale in India only and can be taken only under the advice and guidance of a registered medical practitioner.
Provides a new lease of life to Indian patients of inflammatory arthritis, who did not have access to this revolutionary therapy, so far.
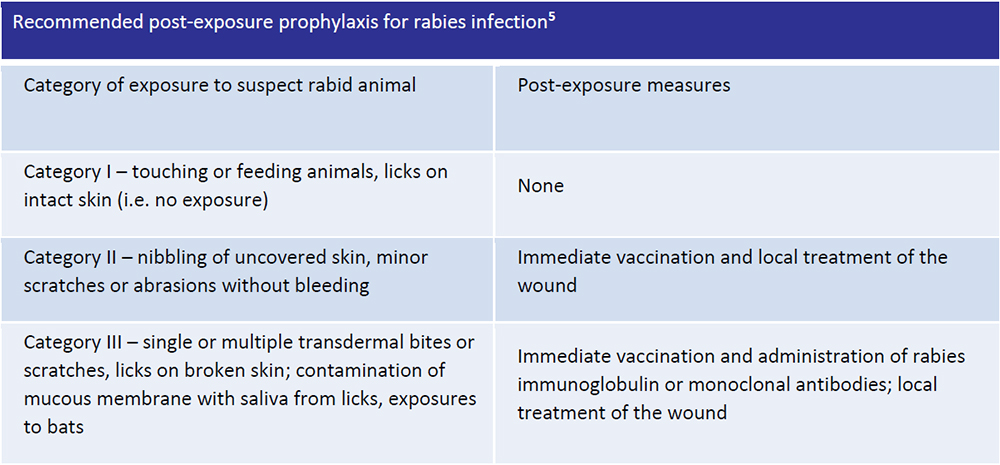
All the cases of rabies exposure should be treated immediately for the prevention of of clinical symptoms and death.
Post-exposure prophylaxis consists of:
Although two types of RIGs (HRIGs and ERIGs) are available, they have the following drawbacks:
1. HRIG:1
2. ERIG:1
Other limitations of HRIG and ERIG7
TwinrabTM is a cocktail of two anti-rabies monoclonal antibodies, indicated for the post-exposure prophylaxis (PEP) of contact with a rabid or suspected rabid animal.
Twinrab™ is an equipotent mixture of two monoclonal antibodies i.e. docaravimab (MAb 62-71-3) and miromavimab (MAb M777-16-3) binding to two distinct sites on the Rabies virus.
The hybridomas were sourced from the following WHO collaborating centres through NIBSC, United Kingdom:
The individual monoclonal antibodies present in the Twinrab™ cocktail mixture were found to neutralize in vitro, various rabies and rabies related viruses such as (CVS 11, SAD B19, PV, Kelev, European Fox, Dog Turkey, Dog Ethiopia, Dog India, Dog Mexico, Wolf Sarajevo, Bobcat-USA, EBLV 1, EBLV 2, East European fox, Polar fox, Dog Azerbaijan, Dog Nepal).
In various in vivo studies, the antibody cocktail was found to neutralize rabies virus isolates (CVS11, Mexican (2004), Thai (2006), Indian (2008) canine variants and Texas Fox 393 rabies virus). In still another in vivo study, the cocktail was also found to neutralize rabies virus isolates from Dog, Canine, Human, and Bovine sources isolated from southern parts of India.
TwinrabTM is indicated for post exposure prophylaxis in individuals with suspected rabies exposure. Twinrab™ must always be used in combination with Rabies vaccine as part of post exposure prophylaxis in line with the recommendation of World Health Organization (WHO).
The recommended dose of TwinrabTM is 40 IU per kg of body weight. TwinrabTM is administered through infiltration around the wounds / scratches and by intramuscular injection.The entire Twinrab™ dose, or as much as anatomically possible (avoiding possible compartment syndrome), should be infiltrated carefully into or as close as possible to the wound(s) or exposure sites.
Zydus state of art vaccine manufacturing facilities are located at two different locations ( Moraiya and Changodar) in Ahmedabad. The vaccine facility located at Changodar is engaged in development and manufacturing of various vaccine products .The vaccine facility located at Moriaya site is engaged in manufacturing of Rabies vaccine (Human) and had received WHO pre-qualification and registered Rabies vaccine (Human) in different countries for export supplies.
Zydus had developed and received Marketing Authorization for various vaccine products like Rabies Vaccine (Human), Typhoid polysaccharide vaccine, Typhoid Vi conjugate vaccine, Inactivated Influenza Vaccine (Tetravalent & Trivalent), Measles containing live attenuated vaccines (Measles vaccine Measles & Rubella vaccine, Measles, Mumps and Rubella vaccine), Varicella vaccine and other combination vaccine like Td vaccine and Pentavalent vaccine. etc. Zydus has indigenously developed, manufactured and launched India's first Tetravalent Inactivated Influenza vaccine- VaxiFlu TM – 4.
During COVID pandemic, Zydus also developed and launched the World’s 1st and only human plasmid DNA vaccine against SARS-CoV-2 under brand name ZyCoV-D and in course also established the plasmid DNA Vaccine platform in the country.
Zydus has a rich pipeline of new vaccine products like Recombinant Hepatitis E, Inactivated Hepatitis A, Human papillomavirus (rDNA), Bivalent Typhoid and Paratyphoid A vaccine, Inactivated Chikungunya vaccine , Recombinant Varicella Vaccine with an aim to cater to the requirements of both India and export markets.
| S.no | Name of Vaccine | Development Phase |
| 1 | Recombinant Hepatitis E Vaccine (Adsorbed) | In Phase II Clinical trial |
| 2 | Inactivated Hepatitis A Vaccine (Adsorbed) | Phase I completed |
| 3 | Measles, Mumps, Rubella and Varicella Vaccine (Live Attenuated ), (Freeze Dried) | Phase I completed |
| 4 | Bivalent Typhoid and Paratyphoid A Conjugate vaccine | Phase I to be initiated |
| 5 | Inactivated Chikungunya Vaccine (Adsorbed) | PCT to be initiated |
| 6 | Human papillomavirus vaccine (rDNA), Nonavalent | PCT to be initiated |
| 7 | Recombinant Varicella Vaccine | Product under development |

ZyCoV-D is a needle-free COVID vaccine . Approved in a two-dose regimen for 12 years and above for primary vaccination and as heterologous booster (third) dose to individuals aged ≥18 years after 6 months of primary vaccination (two doses of either COVAXIN or COVISHIELD).
Zydus acknowledges the support of National Biopharma Mission, BIRAC, Department of Biotechnology, Government of India for funding the development, ICMR-National Institute of Virology, Pune for conduct of NHP Challenge study and PharmaJet Inc., Golden, CO, USA for providing PharmaJet® Tropis® Needle-Free Injection System (NFIS) for vaccine delivery. We also thank all the researchers, clinical trial investigators, volunteers and regulatory agencies including the office of the Drug Controller General of India (DCGI) who supported us in the development of ZyCoV-D.
The vaccine provides protection from the four influenza viruses- H1N1, H3N2, Type B (B/Victoria lineage) and Type B (B/Yamagata lineage)

A global knowledge sharing forum on innovation - The Ramanbhai Foundation International Research Symposium
The Ramanbhai Foundation International Symposium is a biannual series of symposia devoted to the discussion of new trends in the pharmaceutical industry with a view to promoting scientific excellence in drug discovery and development.
Ramanbhai Foundation is named after a pathfinder, Late Mr. Ramanbhai B. Patel, who had dedicated his life to the quest of knowledge, as an academician, entrepreneur and a research scientist. He believed that new paths would surely open up if one has the creative will to discover it.
For more than a decade, the Ramanbhai Foundation (RBF) international symposium has been bringing together experts from both the academia and industry across the world to share their insights on the latest developments in pharmaceutical research. Internationally acclaimed researchers converge to address the various aspects related to New Drug Discovery - with a focus on diabetes, cardiometabolic diseases, NASH, inflammation and infectious diseases. The keynote addresses over the past few years have been delivered by the Nobel Laureates and Research Scientists of international acclaim. The symposium provides an insight into various aspects of drug discovery had an eminent panel of speakers and nearly 500 delegates from India and abroad participated in the symposium.
To know more about the RBF Symposium and/or to participate, please visit www.rbfsymposium.net

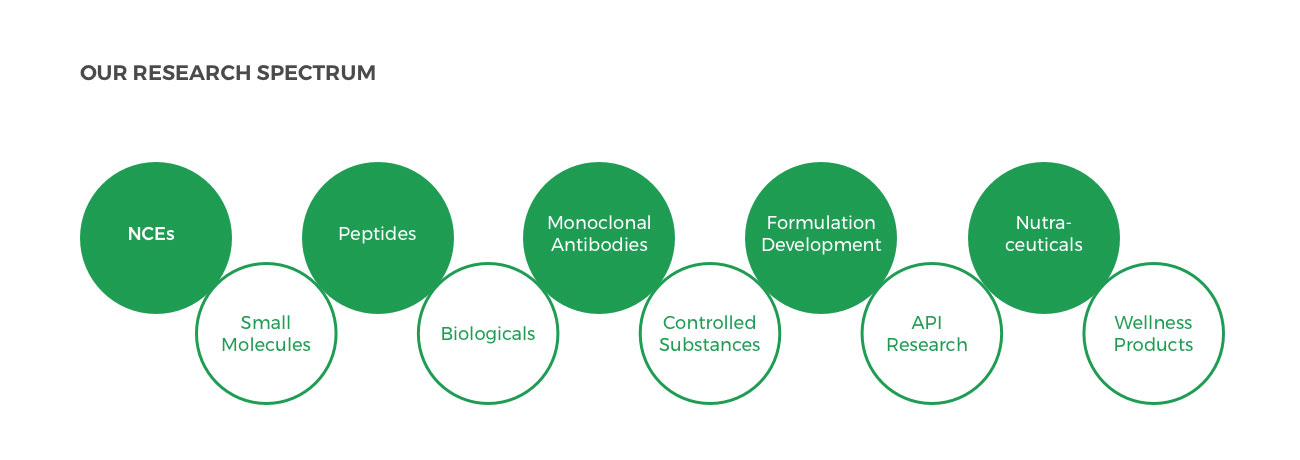
Zydus is constantly exploring opportunities in pharma research under these specific categories:
If you wish to partner with us, you may reach out to -
Business Development
Zydus Research Centre
Sarkhej-Bavla N.H. No. 8A
Moraiya, Ahmedabad – 382210
Gujarat, India.
Phone: +91-2717-665555
Fax: +91-2717-665353